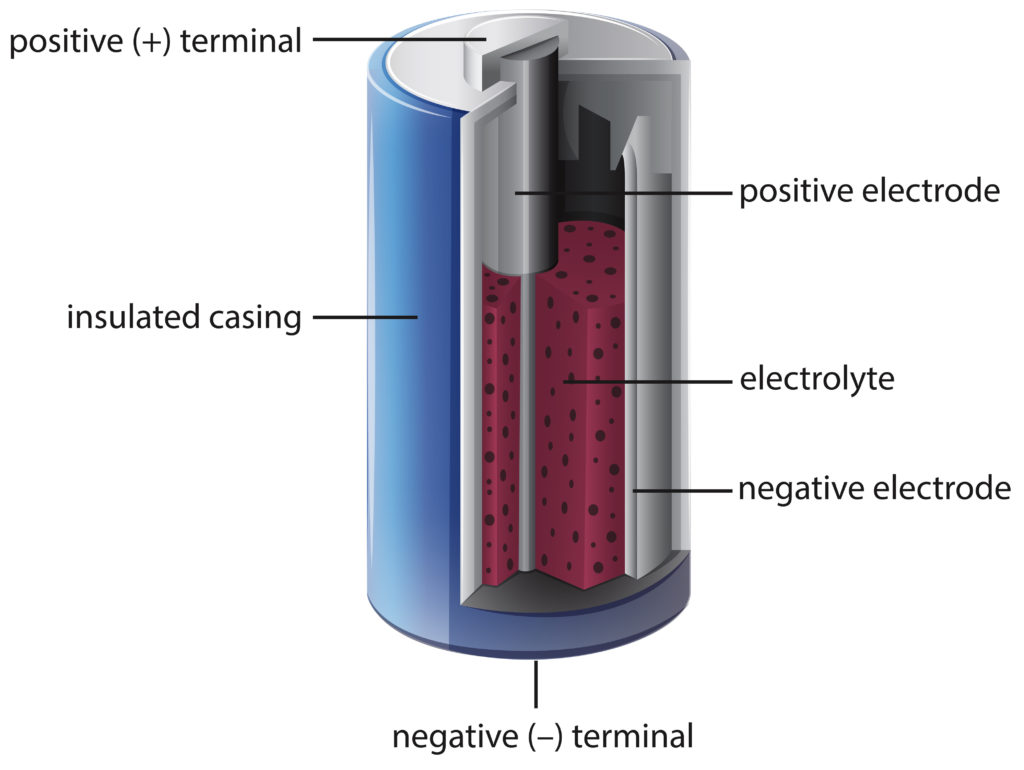
A battery works by converting stored chemical energy into electrical energy through a chemical reaction. This reaction generates electrons.
Batteries are essential power sources for various devices, from smartphones to cars. Understanding how batteries work is crucial for optimizing their performance and longevity. The basic concept involves the conversion of chemical energy into electrical energy. This process occurs through a chemical reaction within the battery that releases electrons.
The movement of these electrons creates an electrical current that powers the connected device. By delving into the inner workings of batteries, we can appreciate the technology that drives our modern world and explore ways to enhance energy storage capabilities for the future.
Credit: www.science-sparks.com
Contents
The Basics of Battery Operation
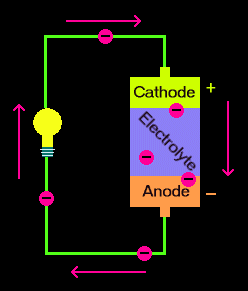
At its core, a battery is a device that converts chemical energy into electrical energy through electrochemical reactions. These reactions involve the transfer of electrons from one material to another, creating a flow of electric current. Let’s break down the main components and processes involved in this transformation.
Key Components of a Battery
- Electrodes:
- Anode (Negative Electrode): The anode is the electrode where oxidation occurs, meaning it loses electrons during the electrochemical reaction. In many batteries, the anode is made of materials like lithium, zinc, or lead.
- Cathode (Positive Electrode): The cathode is the electrode where reduction occurs, meaning it gains electrons. Common cathode materials include manganese dioxide, nickel oxide, and lead dioxide.
- Electrolyte:
- The electrolyte is a medium that allows ions to move between the anode and cathode. It can be a liquid, gel, or solid substance. The electrolyte must be conductive to ions but not to electrons to prevent short-circuiting within the battery.
- Separator:
- The separator is a physical barrier that prevents the anode and cathode from coming into direct contact, which would cause a short circuit. It allows the flow of ions through the electrolyte while keeping the electrodes apart.
Credit: www.qrg.northwestern.edu
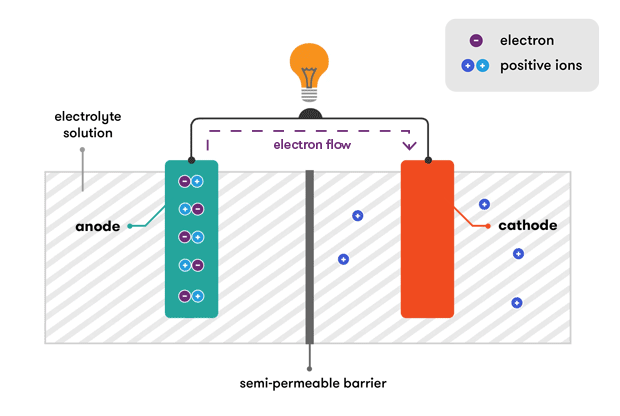
How a Battery Generates Electricity
The process of generating electricity in a battery involves the following steps:
- Chemical Reaction:
- When the battery is connected to a circuit, a chemical reaction occurs between the anode and the electrolyte, releasing electrons from the anode material.
- Electron Flow:
- The released electrons flow from the anode through the external circuit, providing electrical power to connected devices, and then return to the cathode.
- Ion Movement:
- Simultaneously, positively charged ions from the anode travel through the electrolyte to the cathode to balance the charge. The electrolyte facilitates this movement of ions.
- Reduction at Cathode:
- At the cathode, the electrons that traveled through the external circuit combine with the incoming positive ions, resulting in a reduction reaction that completes the circuit.
This continuous flow of electrons through the external circuit and ions within the battery generates a steady supply of electrical energy until the reactants are depleted.
Types of Batteries
There are several types of batteries, each designed for specific applications and with distinct advantages and disadvantages. Here are the most common types:
- Primary Batteries:
- Alkaline Batteries: Widely used in household items like remote controls and flashlights. They are disposable and cannot be recharged.
- Lithium Batteries: Known for their high energy density and long shelf life, used in devices like cameras and smoke detectors.
- Secondary Batteries (Rechargeable):
- Lithium-Ion Batteries: Commonly used in smartphones, laptops, and electric vehicles. They offer high energy density and can be recharged multiple times.
- Nickel-Metal Hydride (NiMH) Batteries: Often used in power tools and hybrid vehicles, they provide good performance and are environmentally friendly.
- Lead-Acid Batteries: Used in automotive and backup power applications, they are robust and cost-effective but heavier and have lower energy density compared to other rechargeable batteries.
Innovations in Battery Technology
Advancements in battery technology are continually being made to address the growing demand for more efficient, longer-lasting, and environmentally friendly energy storage solutions. Some notable innovations include:
- Solid-State Batteries:
- These batteries use a solid electrolyte instead of a liquid one, offering higher energy density, improved safety, and longer lifespan.
- Flow Batteries:
- Flow batteries store energy in liquid electrolytes contained in external tanks, making them suitable for large-scale energy storage applications like grid storage.
- Sodium-Ion Batteries:
- An emerging technology that uses sodium instead of lithium, offering a more abundant and potentially cheaper alternative for energy storage.
Credit: www.science.org.au
Frequently Asked Questions
Here are some FAQs about the battery mechanism –
What Are The Different Types Of Batteries?
There are several types of batteries, including alkaline, lithium-ion, lead-acid, nickel-cadmium, and nickel-metal hydride batteries. Each type has its own unique characteristics and applications.
How Long Do Batteries Last?
The lifespan of a battery depends on various factors such as usage, type, and quality. Generally, batteries can last anywhere from a few hours to several years before needing to be replaced or recharged.
Can Batteries Be Recharged?
Yes, some batteries are rechargeable, such as lithium-ion batteries. These batteries can be recharged multiple times by applying an electric current to reverse the chemical reaction that occurs during discharge.
How Should Batteries Be Disposed Of?
Batteries should be disposed of properly to prevent environmental harm. Many communities have designated recycling centers or battery disposal locations. It is important to follow local regulations and guidelines for safe disposal.
Conclusion
Understanding the inner workings of a battery is essential for everyday life. By learning how chemical reactions create electrical energy, we gain insight into a fundamental aspect of modern technology. With this knowledge, we can make informed decisions about battery usage and contribute to a more sustainable future.